Study about Aculief
Introduction
Tension type headache (TTH) is a common pathological condition that affects over 75% of theworld’s population [1].
The severity of the condition varies significantly and only 16% of thoseaffected receive treatment e.g. hydration, relaxation, sleep, and meditation [2].
The Aculief deviceis a non-significant medical device, designed specifically to apply pressure at the LI4 acupoint are cognized location in the treatment of headache.
Primary objective is was to determine if average intensity of head pain over a 2-week period, measured by the Visual Analog Scale (VAS), decreases with the utilization of Aculief acupressure device in patients diagnosed with frequent tension type headache.
Secondary objective was to determine if the Aculief acupressure device helps decrease pain medication usage in subjects using medication specifically for pain related to their headache
Study Summary
A single site, open, single-blinded, randomized 2×2 crossover study was conducted at “Institute for Regenerative Medicine and Clinical Research, Pasadena, CA” for a period of 35 days after being approved by a national institutional review board (IRB).
Total of 91 patients (age ≥ 18 years) with the active diagnostic criteria of frequent tension type headaches listed in “International Headache Society v3.0” were included in the study after being agreed to clinical trial with verified documentation of Informed Consent, HIPPA, and California Bill of Rights.
Exclusion criteria included the patients with overlapping symptoms of other headaches, those who were enrolled in another medical study, under any kind of litigation, pregnant women and those with no confirmed diagnosis.
The participants were identified and recruited from the utilization of “Synovation Medical Group’s Electronic Medical Record software” by P.I and/or sub-I through listed preferred method of communication. Initially, 122 participants were recruited on the basis of inclusion criteria.However, final data was collected from 91 participants: 48 (52.7%) in AB sequence (Aculief phase 1) and 43 (47.3%) in BA sequence (Aculief phase 2).
Most of the participants were male (51.6%), 21-30 years of age and women 45 (49.5%; Table-1). 31 participants left the study.
During phase 1, the subjects in group AB received the device while those in group BA received only SMC.
During washout period (one week after phase 1), the participants received neither the device nor the SMC.
During phase 2, the subjects in group AB received SMC and those in group BA received the device.
Adverse Events/Complications
There were zero adverse events or complications recorded throughout the entire trial.
Device Failure
There were zero device failures recorded throughout the entire trial.
Data Collection
Data was collected from pain journals, and medication use journals. The pain journals yielded VAS pain intensity at 30-minute intervals for each headache experienced by each subject.
This was then used to extrapolate headache intensity, duration, and frequency. The medication usage journals were used to determine medication use by each subject throughout the duration of the headaches.
Based on the study protocol, each subject underwent 2-weeks of baseline data as a routine standard medical care and 2- weeks of data that included the Aculief device plus standard medical of care. The mean headache intensity, duration, frequency and medication usage for each subject were determined for each 2-week period.
Statistical Methods
ANOVA was used to analyze the data collected from pain journals by subjects enrolled in the clinical trial. A sample size calculation using SPSS software was carried out using standard parameters of a power of 80%, and a significance level of 0.05, and a total of 41 subjects were determined to be needed for enrollment in the study to detect a moderate effect size of a 30% decrease in pain level, measured by VAS.
Device Instructions
Aculief was instructed to be placed between thumb and forefinger on either hand as pictured. The device was instructed to be used immediately at the onset of headache. It was to be worn throughout the episode and be documented in the appropriate sections in the pain journals provided the time and duration it was worn and synchronize it to the timing and duration of headache, as well as the medication usage (if any) used as well.
Study Results
The study revealed that the subjects in group A reported less VAS score and less number of medications as compared to those in group B (table-6).
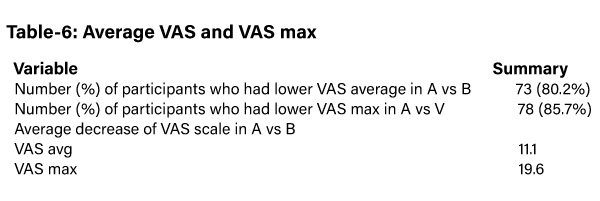
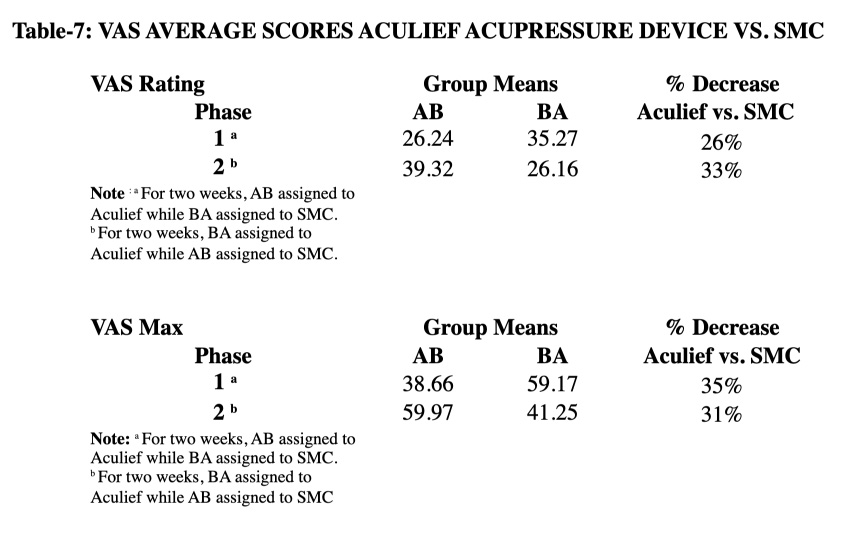
In phase 1, the subjects in AB were assigned to Aculief and they showed less VAS score as compared to BA which was assigned to standard medical care (SMC) (table-7).
On the contrast, AB was assigned to SMC and BA was assigned to Aculief in phase 2. So, the subjects in BA in phase 2 showed less VAS score as compared to those in AB.
The same results were reported with VAS Max (table-7).
Conclusion
This study reveals that Aculief significantly decreased VAS score as compared to SMC. In phase 1, average number of pain episodes, average VAS score and average VAS Max were significantly less with Aculief device (p= 0.000, p= 0.004, p= 0.000, respectively; tables 6 & 7).
Although not statistically significant, but medication use was reduced in the participants in group with the Aculief device as compared to those in SMC group.
In phase 2, the findings were consistent with phase 1in terms of average VAS score, average VAS Max and medication use; however, findings were non-consistent with phase 1 in terms of average number of pain episodes.
This demonstrates Aculief significantly reduced average VAS score and average VAS Max in the patients with tension type headache.
Considering the above results, it is necessary to mention why this study is important in terms of its endpoints. In the present study, VAS scale improvement was averaging around 30 which showed a significant improvement in the pain symptoms where the patients feel clinically improved.
In this regard, several studies have mentioned 30 mm improvement (even less than 30mm) on 0-100 mm VAS as significant. Tubach et al. [3] reported that the patients with severe hipOA symptoms felt clinically improved with NSAID therapy when their decrease in pain exceeded 36.6 mm on the 0-100 mm VAS.
It was termed as “minimal clinically important improvement (MCII). Similarly, they reported that the patients with less pain felt clinically improved when their decrease in pain exceeded 10.8 mm on the VAS. In another study by Tashjian et al. [4] reported minimal clinically important difference of 1.4 cm on 10 cm VAS measuring pain in the patients with rotator cuff disease.
In a study, Lee et al. [5] reported that 30 mm mean reduction on 100-mm VAS was clinically important difference in pain severity where the patients felt adequate pain control in conditions with acute pain. In this context, the Aculief Acupressure device led to 30 mm reduction in VAS in the present study, promising that it can control pain well in the patients with tension type headaches.
Similarly, the participants included in the group with the Aculief Acupressure device revealed reduced use of medication as compared to those in the group with SMC. Although the reduction in medication use by Aculief group was not statistically significant; however, any reduction in medication use by the study group supports the Aculief use as compared to SMC, and encourages more studies to be conducted to evaluate this issue in order to provide better treatment to the patients with tension type headaches.
References
1. Schulman EA. Overview of Tension Type Headache. Curr Pain Headache Rep. 2001;5:455.
2. Schulman EA. Overview of Tension Type Headache. Curr Pain Headache Rep. 2001;5:454.
3. Tubach F, Ravaud P, Baron G, Falissard B, Loge art I, Bellamy N, Bombardier C, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis 2005; 64:29-33.
4. Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences(MCID) and patient acceptable symptomatic state(PASS) for visual analog scales(VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg 2009; 18(6):927-32.
5. Lee JS, Hobden E, Stiell IG, Wells GA. Clinically Important Change in the Visual Analog Scale after Adequate Pain Control. Acad Emerg Med 2003;10(10):1128-30